The effectiveness of various chemical disinfectants is reflected in the terms used to describe them. Chemical disinfectants are grouped by the power of their activity, with each category reflecting the types of microbes and viruses its component disinfectants are effective against. High-level germicides have the ability to kill vegetative cells, fungi, viruses, and endospores, leading to sterilization, with extended use. Intermediate-level germicides, as their name suggests, are less effective against endospores and certain viruses, and low-level germicides kill only vegetative cells and certain enveloped viruses, and are ineffective against endospores.
However, several environmental conditions influence the potency of an antimicrobial agent and its effectiveness. For example, length of exposure is particularly important, with longer exposure increasing efficacy. Similarly, the concentration of the chemical agent is also important, with higher concentrations being more effective than lower ones. Temperature, pH, and other factors can also affect the potency of a disinfecting agent.
One method to determine the effectiveness of a chemical agent includes swabbing surfaces before and after use to confirm whether a sterile field was maintained during use. Additional tests are described in the sections that follow. These tests allow for the maintenance of appropriate disinfection protocols in clinical settings, controlling microbial growth to protect patients, health-care workers, and the community.
The effectiveness of a disinfectant or antiseptic can be determined in a number of ways. Historically, a chemical agent’s effectiveness was often compared with that of phenol, the first chemical agent used by Joseph Lister. In 1903, British chemists Samuel Rideal (1863–1929) and J. T. Ainslie Walker (1868–1930) established a protocol to compare the effectiveness of a variety of chemicals with that of phenol, using as their test organisms Staphylococcus aureus (a gram-positive bacterium) and Salmonella enterica serovar Typhi (a gram-negative bacterium). They exposed the test bacteria to the antimicrobial chemical solutions diluted in water for 7.5 minutes. They then calculated a phenol coefficient for each chemical for each of the two bacteria tested. A phenol coefficient of 1.0 means that the chemical agent has about the same level of effectiveness as phenol. A chemical agent with a phenol coefficient of less than 1.0 is less effective than phenol. An example is formalin, with phenol coefficients of 0.3 (S. aureus) and 0.7 (S. enterica serovar Typhi). A chemical agent with a phenol coefficient greater than 1.0 is more effective than phenol, such as chloramine, with phenol coefficients of 133 and 100, respectively. Although the phenol coefficient was once a useful measure of effectiveness, it is no longer commonly used because the conditions and organisms used were arbitrarily chosen.
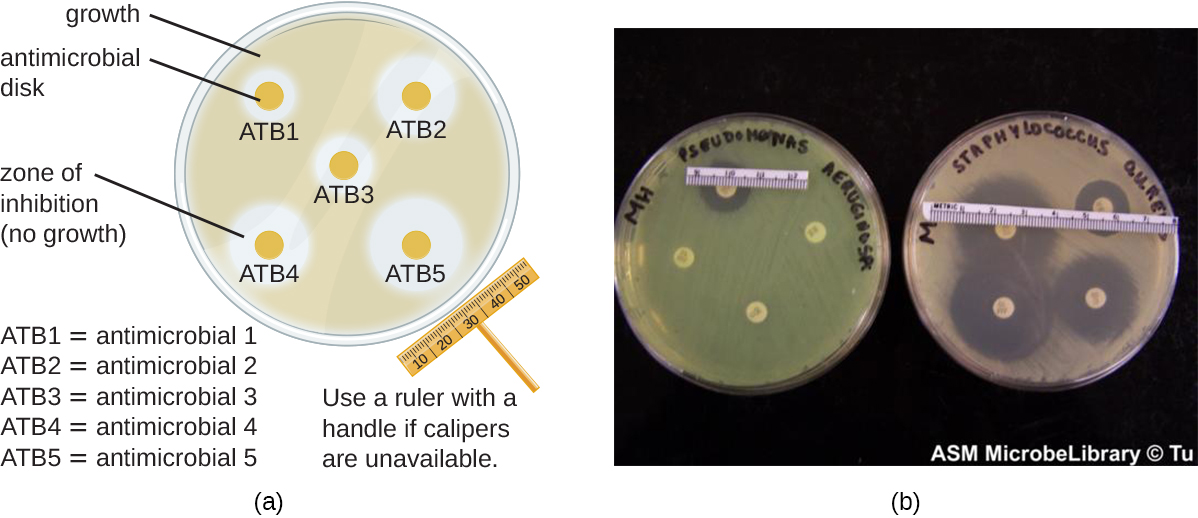
The disk-diffusion method involves applying different chemicals to separate, sterile filter paper disks ([link]). The disks are then placed on an agar plate that has been inoculated with the targeted bacterium and the chemicals diffuse out of the disks into the agar where the bacteria have been inoculated. As the “lawn” of bacteria grows, zones of inhibition of microbial growth are observed as clear areas around the disks. Although there are other factors that contribute to the sizes of zones of inhibition (e.g., whether the agent is water soluble and able to diffuse in the agar), larger zones typically correlate to increased inhibition effectiveness of the chemical agent. The diameter across each zone is measured in millimeters.

Other methods are also used for measuring the effectiveness of a chemical agent in clinical settings. The use-dilution test is commonly used to determine a chemical’s disinfection effectiveness on an inanimate surface. For this test, a cylinder of stainless steel is dipped in a culture of the targeted microorganism and then dried. The cylinder is then dipped in solutions of disinfectant at various concentrations for a specified amount of time. Finally, the cylinder is transferred to a new test tube containing fresh sterile medium that does not contain disinfectant, and this test tube is incubated. Bacterial survival is demonstrated by the presence of turbidity in the medium, whereas killing of the target organism on the cylinder by the disinfectant will produce no turbidity.
The Association of Official Agricultural Chemists International (AOAC), a nonprofit group that establishes many protocol standards, has determined that a minimum of 59 of 60 replicates must show no growth in such a test to achieve a passing result, and the results must be repeatable from different batches of disinfectant and when performed on different days. Disinfectant manufacturers perform use-dilution tests to validate the efficacy claims for their products, as designated by the EPA.
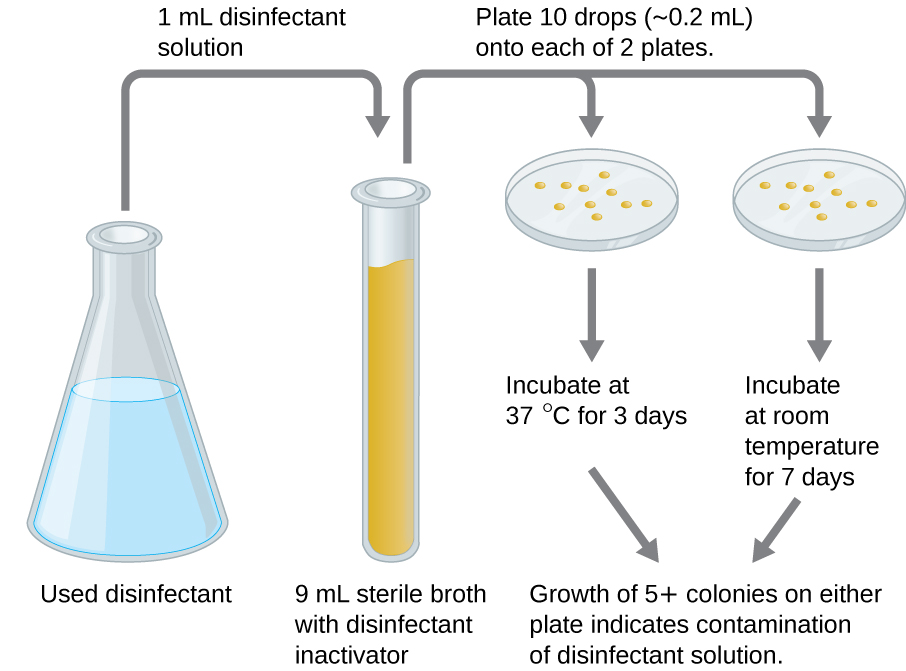
An in-use test can determine whether an actively used solution of disinfectant in a clinical setting is microbially contaminated ([link]). A 1-mL sample of the used disinfectant is diluted into 9 mL of sterile broth medium that also contains a compound to inactivate the disinfectant. Ten drops, totaling approximately 0.2 mL of this mixture, are then inoculated onto each of two agar plates. One plate is incubated at 37 °C for 3 days and the other is incubated at room temperature for 7 days. The plates are monitored for growth of microbial colonies. Growth of five or more colonies on either plate suggests that viable microbial cells existed in the disinfectant solution and that it is contaminated. Such in-use tests monitor the effectiveness of disinfectants in the clinical setting.
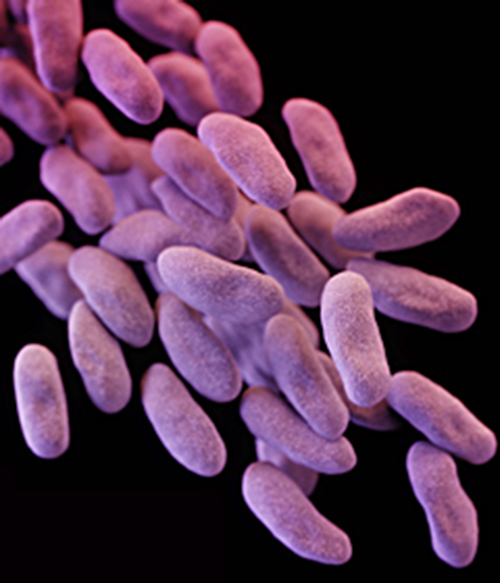
Despite antibiotic treatment, Roberta’s symptoms worsened. She developed pyelonephritis, a severe kidney infection, and was rehospitalized in the intensive care unit (ICU). Her condition continued to deteriorate, and she developed symptoms of septic shock. At this point, her physician ordered a culture from her urine to determine the exact cause of her infection, as well as a drug sensitivity test to determine what antibiotics would be effective against the causative bacterium. The results of this test indicated resistance to a wide range of antibiotics, including the carbapenems, a class of antibiotics that are used as the last resort for many types of bacterial infections. This was an alarming outcome, suggesting that Roberta’s infection was caused by a so-called superbug: a bacterial strain that has developed resistance to the majority of commonly used antibiotics. In this case, the causative agent belonged to the carbapenem-resistant Enterobacteriaceae (CRE), a drug-resistant family of bacteria normally found in the digestive system ([link]). When CRE is introduced to other body systems, as might occur through improperly cleaned surgical instruments, catheters, or endoscopes, aggressive infections can occur.
CRE infections are notoriously difficult to treat, with a 40%–50% fatality rate. To treat her kidney infection and septic shock, Roberta was treated with dialysis, intravenous fluids, and medications to maintain blood pressure and prevent blood clotting. She was also started on aggressive treatment with intravenous administration of a new drug called tigecycline, which has been successful in treating infections caused by drug-resistant bacteria.
After several weeks in the ICU, Roberta recovered from her CRE infection. However, public health officials soon noticed that Roberta’s case was not isolated. Several patients who underwent similar procedures at the same hospital also developed CRE infections, some dying as a result. Ultimately, the source of the infection was traced to the duodenoscopes used in the procedures. Despite the hospital staff meticulously following manufacturer protocols for disinfection, bacteria, including CRE, remained within the instruments and were introduced to patients during procedures.
Go back to the previous Clinical Focus box.
Carbapenem-resistant Enterobacteriaceae infections due to contaminated endoscopes have become a high-profile problem in recent years. Several CRE outbreaks have been traced to endoscopes, including a case at Ronald Reagan UCLA Medical Center in early 2015 in which 179 patients may have been exposed to a contaminated endoscope. Seven of the patients developed infections, and two later died. Several lawsuits have been filed against Olympus, the manufacturer of the endoscopes. Some claim that Olympus did not obtain FDA approval for design changes that may have led to contamination, and others claim that the manufacturer knowingly withheld information from hospitals concerning defects in the endoscopes.
Lawsuits like these raise difficult-to-answer questions about liability. Invasive procedures are inherently risky, but negative outcomes can be minimized by strict adherence to established protocols. Who is responsible, however, when negative outcomes occur due to flawed protocols or faulty equipment? Can hospitals or health-care workers be held liable if they have strictly followed a flawed procedure? Should manufacturers be held liable—and perhaps be driven out of business—if their lifesaving equipment fails or is found defective? What is the government’s role in ensuring that use and maintenance of medical equipment and protocols are fail-safe?
Protocols for cleaning or sterilizing medical equipment are often developed by government agencies like the FDA, and other groups, like the AOAC, a nonprofit scientific organization that establishes many protocols for standard use globally. These procedures and protocols are then adopted by medical device and equipment manufacturers. Ultimately, the end-users (hospitals and their staff) are responsible for following these procedures and can be held liable if a breach occurs and patients become ill from improperly cleaned equipment.
Unfortunately, protocols are not infallible, and sometimes it takes negative outcomes to reveal their flaws. In 2008, the FDA had approved a disinfection protocol for endoscopes, using glutaraldehyde (at a lower concentration when mixed with phenol), o-phthalaldehyde, hydrogen peroxide, peracetic acid, and a mix of hydrogen peroxide with peracetic acid. However, subsequent CRE outbreaks from endoscope use showed that this protocol alone was inadequate.
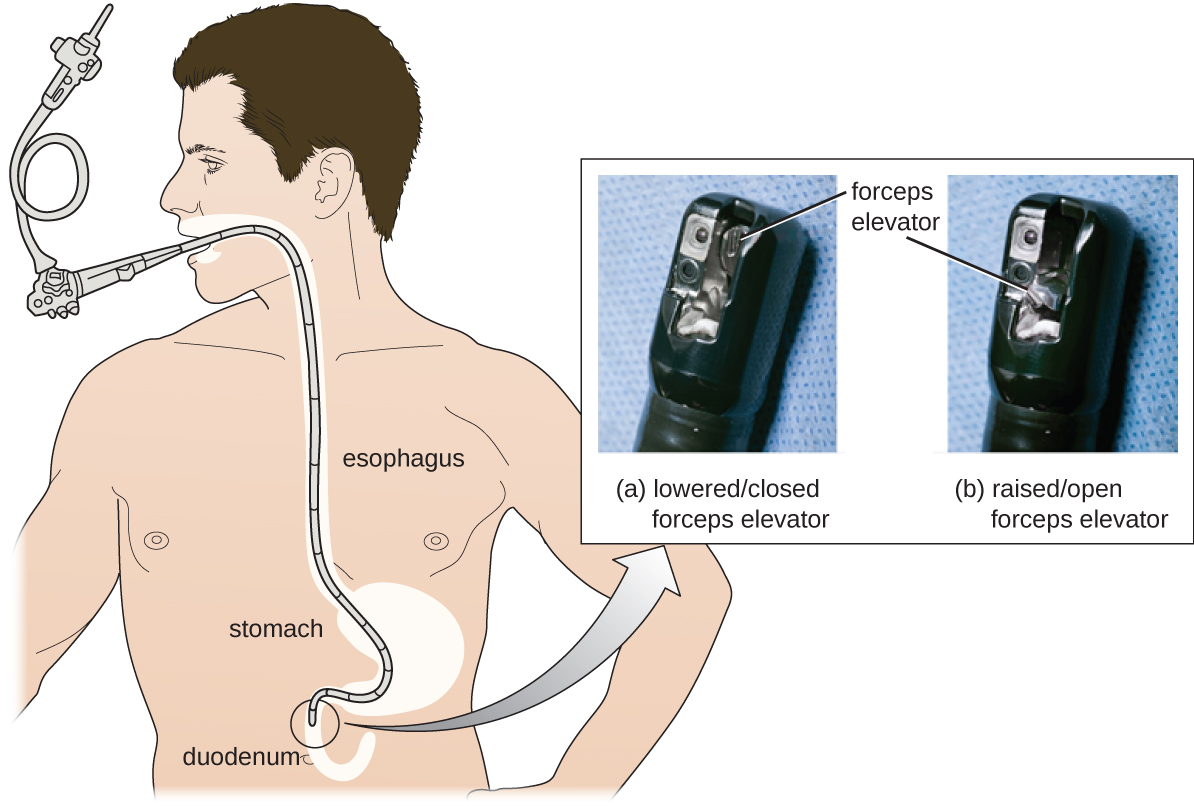
As a result of CRE outbreaks, hospitals, manufacturers, and the FDA are investigating solutions. Many hospitals are instituting more rigorous cleaning procedures than those mandated by the FDA. Manufacturers are looking for ways to redesign duodenoscopes to minimize hard-to-reach crevices where bacteria can escape disinfectants, and the FDA is updating its protocols. In February 2015, the FDA added new recommendations for careful hand cleaning of the duodenoscope elevator mechanism (the location where microbes are most likely to escape disinfection), and issued more careful documentation about quality control of disinfection protocols ([link]).
There is no guarantee that new procedures, protocols, or equipment will completely eliminate the risk for infection associated with endoscopes. Yet these devices are used successfully in 500,000–650,000 procedures annually in the United States, many of them lifesaving. At what point do the risks outweigh the benefits of these devices, and who should be held responsible when negative outcomes occur?
Which type of test is used to determine whether disinfectant solutions actively used in a clinical setting are being used correctly?
C
The effectiveness of chemical disinfectants has historically been compared to that of which of the following?
A
Which of the following refers to a germicide that can kill vegetative cells and certain enveloped viruses but not endospores?
C
If a chemical disinfectant is more effective than phenol, then its phenol coefficient would be ________ than 1.0.
greater
If used for extended periods of time, ________ germicides may lead to sterility.
high-level
In the disk-diffusion assay, a large zone of inhibition around a disk to which a chemical disinfectant has been applied indicates ________ of the test microbe to the chemical disinfectant.
susceptibility or sensitivity
Why were chemical disinfectants once commonly compared with phenol?
Why is length of exposure to a chemical disinfectant important for its activity?
What are some advantages of use-dilution and in-use tests compared with the disk-diffusion assay?

You can also download for free at http://cnx.org/contents/e42bd376-624b-4c0f-972f-e0c57998e765@5.3
Attribution: