
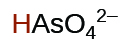

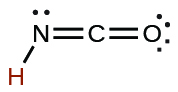

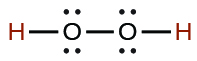

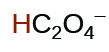




| Ionization Constants of Weak Acids | |||
|---|---|---|---|
| Acid | Formula | Ka at 25 °C | Lewis Structure |
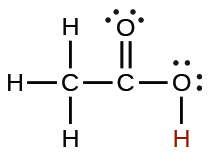
| acetic | CH3CO2H | 1.8 10−5 |  |
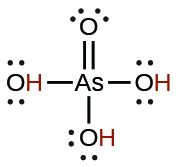
| arsenic | H3AsO4 | 5.5 10−3 |  |
 |
1.7 10−7 | ||
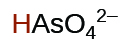 |
5.1 10−12 | ||
| arsenous | H3AsO3 | 5.1 10−10 |  |
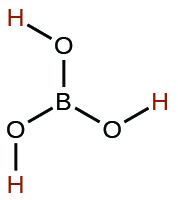
| boric | H3BO3 | 5.4 10−10 |  |
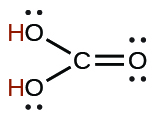
| carbonic | H2CO3 | 4.3 10−7 |  |
 |
4.7 10−11 | ||
| cyanic | HCNO | 2 10−4 | 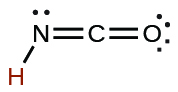 |
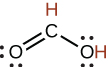
| formic | HCO2H | 1.8 10−4 |  |
| hydrazoic | HN3 | 2.5 10−5 |  |
| hydrocyanic | HCN | 4.9 10−10 | |
| hydrofluoric | HF | 3.5 10−4 | |
| hydrogen peroxide | H2O2 | 2.4 10−12 | 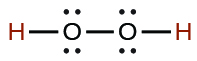 |
| hydrogen selenide | H2Se | 1.29 10−4 | |
| HSe– | 1 10−12 | ||
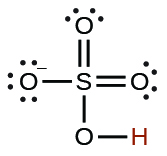
| hydrogen sulfate ion |  |
1.2 10−2 |  |
| hydrogen sulfide | H2S | 8.9 10−8 | |
| HS– | 1.0 10−19 | ||
| hydrogen telluride | H2Te | 2.3 10−3 | |
| HTe– | 1.6 10−11 | ||
| hypobromous | HBrO | 2.8 10−9 | |
| hypochlorous | HClO | 2.9 10−8 | |
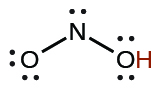
| nitrous | HNO2 | 4.6 10−4 |  |
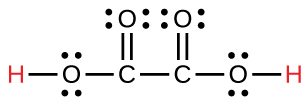
| oxalic | H2C2O4 | 6.0 10−2 |  |
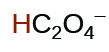 |
6.1 10−5 | ||
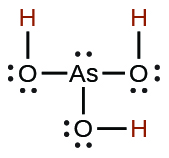
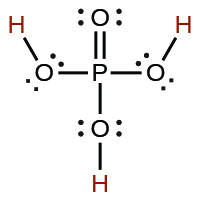
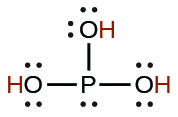
| phosphoric | H3PO4 | 7.5 10−3 |  |
 |
6.2 10−8 | ||
 |
4.2 10−13 | ||
| phosphorous | H3PO3 | 5 10−2 |  |
 |
2.0 10−7 | ||
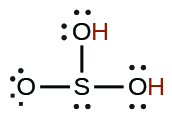
| sulfurous | H2SO3 | 1.6 10−2 |  |
 |
6.4 10−8 | ||

You can also download for free at http://cnx.org/contents/85abf193-2bd2-4908-8563-90b8a7ac8df6@12.1
Attribution: