
By the end of this section, you will be able to:
Do you ever wonder why scientists spend time looking for water on other planets? It is because water is essential to life; even minute traces of it on another planet can indicate that life could or did exist on that planet. Water is one of the more abundant molecules in living cells and the one most critical to life as we know it. Approximately 60–70 percent of your body is made up of water. Without it, life simply would not exist.
The hydrogen and oxygen atoms within water molecules form polar covalent bonds. The shared electrons spend more time associated with the oxygen atom than they do with hydrogen atoms. There is no overall charge to a water molecule, but there is a slight positive charge on each hydrogen atom and a slight negative charge on the oxygen atom. Because of these charges, the slightly positive hydrogen atoms repel each other and form the unique shape seen in [link]. Each water molecule attracts other water molecules because of the positive and negative charges in the different parts of the molecule. Water also attracts other polar molecules (such as sugars), forming hydrogen bonds. When a substance readily forms hydrogen bonds with water, it can dissolve in water and is referred to as hydrophilic (“water-loving”). Hydrogen bonds are not readily formed with nonpolar substances like oils and fats ([link]). These nonpolar compounds are hydrophobic (“water-fearing”) and will not dissolve in water.

The hydrogen bonds in water allow it to absorb and release heat energy more slowly than many other substances. Temperature is a measure of the motion (kinetic energy) of molecules. As the motion increases, energy is higher and thus temperature is higher. Water absorbs a great deal of energy before its temperature rises. Increased energy disrupts the hydrogen bonds between water molecules. Because these bonds can be created and disrupted rapidly, water absorbs an increase in energy and temperature changes only minimally. This means that water moderates temperature changes within organisms and in their environments. As energy input continues, the balance between hydrogen-bond formation and destruction swings toward the destruction side. More bonds are broken than are formed. This process results in the release of individual water molecules at the surface of the liquid (such as a body of water, the leaves of a plant, or the skin of an organism) in a process called evaporation. Evaporation of sweat, which is 90 percent water, allows for cooling of an organism, because breaking hydrogen bonds requires an input of energy and takes heat away from the body.
Conversely, as molecular motion decreases and temperatures drop, less energy is present to break the hydrogen bonds between water molecules. These bonds remain intact and begin to form a rigid, lattice-like structure (e.g., ice) ([link]a). When frozen, ice is less dense than liquid water (the molecules are farther apart). This means that ice floats on the surface of a body of water ([link]b). In lakes, ponds, and oceans, ice will form on the surface of the water, creating an insulating barrier to protect the animal and plant life beneath from freezing in the water. If this did not happen, plants and animals living in water would freeze in a block of ice and could not move freely, making life in cold temperatures difficult or impossible.
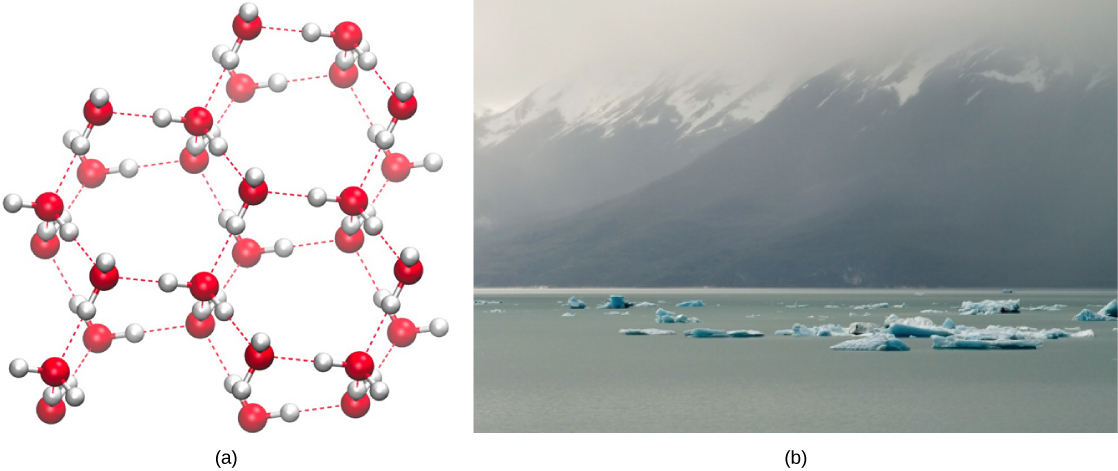
 Click here to see a 3-D animation of the structure of an ice lattice. (credit: image created by Jane Whitney using Visual Molecular Dynamics (VMD) software1)
Click here to see a 3-D animation of the structure of an ice lattice. (credit: image created by Jane Whitney using Visual Molecular Dynamics (VMD) software1)
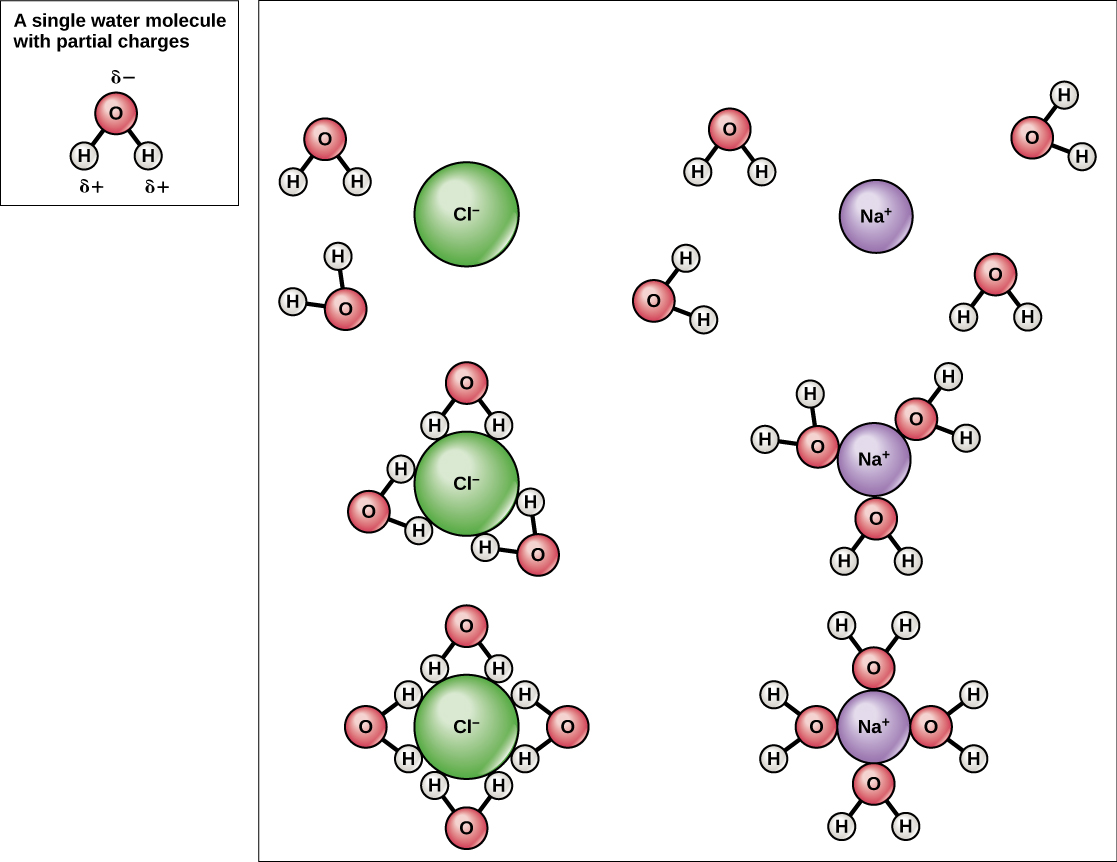
Because water is polar, with slight positive and negative charges, ionic compounds and polar molecules can readily dissolve in it. Water is, therefore, what is referred to as a solvent—a substance capable of dissolving another substance. The charged particles will form hydrogen bonds with a surrounding layer of water molecules. This is referred to as a sphere of hydration and serves to keep the particles separated or dispersed in the water. In the case of table salt (NaCl) mixed in water ([link]), the sodium and chloride ions separate, or dissociate, in the water, and spheres of hydration are formed around the ions. A positively charged sodium ion is surrounded by the partially negative charges of oxygen atoms in water molecules. A negatively charged chloride ion is surrounded by the partially positive charges of hydrogen atoms in water molecules. These spheres of hydration are also referred to as hydration shells. The polarity of the water molecule makes it an effective solvent and is important in its many roles in living systems.
Have you ever filled up a glass of water to the very top and then slowly added a few more drops? Before it overflows, the water actually forms a dome-like shape above the rim of the glass. This water can stay above the glass because of the property of cohesion. In cohesion, water molecules are attracted to each other (because of hydrogen bonding), keeping the molecules together at the liquid-air (gas) interface, although there is no more room in the glass. Cohesion gives rise to surface tension, the capacity of a substance to withstand rupture when placed under tension or stress. When you drop a small scrap of paper onto a droplet of water, the paper floats on top of the water droplet, although the object is denser (heavier) than the water. This occurs because of the surface tension that is created by the water molecules. Cohesion and surface tension keep the water molecules intact and the item floating on the top. It is even possible to “float” a steel needle on top of a glass of water if you place it gently, without breaking the surface tension ([link]).

These cohesive forces are also related to the water’s property of adhesion, or the attraction between water molecules and other molecules. This is observed when water “climbs” up a straw placed in a glass of water. You will notice that the water appears to be higher on the sides of the straw than in the middle. This is because the water molecules are attracted to the straw and therefore adhere to it.
Cohesive and adhesive forces are important for sustaining life. For example, because of these forces, water can flow up from the roots to the tops of plants to feed the plant.
 To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! website.
To learn more about water, visit the U.S. Geological Survey Water Science for Schools: All About Water! website.
The pH of a solution is a measure of its acidity or alkalinity. You have probably used litmus paper, paper that has been treated with a natural water-soluble dye so it can be used as a pH indicator, to test how much acid or base (alkalinity) exists in a solution. You might have even used some to make sure the water in an outdoor swimming pool is properly treated. In both cases, this pH test measures the amount of hydrogen ions that exists in a given solution. High concentrations of hydrogen ions yield a low pH, whereas low levels of hydrogen ions result in a high pH. The overall concentration of hydrogen ions is inversely related to its pH and can be measured on the pH scale ([link]). Therefore, the more hydrogen ions present, the lower the pH; conversely, the fewer hydrogen ions, the higher the pH.
The pH scale ranges from 0 to 14. A change of one unit on the pH scale represents a change in the concentration of hydrogen ions by a factor of 10, a change in two units represents a change in the concentration of hydrogen ions by a factor of 100. Thus, small changes in pH represent large changes in the concentrations of hydrogen ions. Pure water is neutral. It is neither acidic nor basic, and has a pH of 7.0. Anything below 7.0 (ranging from 0.0 to 6.9) is acidic, and anything above 7.0 (from 7.1 to 14.0) is alkaline. The blood in your veins is slightly alkaline (pH = 7.4). The environment in your stomach is highly acidic (pH = 1 to 2). Orange juice is mildly acidic (pH = approximately 3.5), whereas baking soda is basic (pH = 9.0).

Acids are substances that provide hydrogen ions (H+) and lower pH, whereas bases provide hydroxide ions (OH–) and raise pH. The stronger the acid, the more readily it donates H+. For example, hydrochloric acid and lemon juice are very acidic and readily give up H+ when added to water. Conversely, bases are those substances that readily donate OH–. The OH– ions combine with H+ to produce water, which raises a substance’s pH. Sodium hydroxide and many household cleaners are very alkaline and give up OH– rapidly when placed in water, thereby raising the pH.
Most cells in our bodies operate within a very narrow window of the pH scale, typically ranging only from 7.2 to 7.6. If the pH of the body is outside of this range, the respiratory system malfunctions, as do other organs in the body. Cells no longer function properly, and proteins will break down. Deviation outside of the pH range can induce coma or even cause death.
So how is it that we can ingest or inhale acidic or basic substances and not die? Buffers are the key. Buffers readily absorb excess H+ or OH–, keeping the pH of the body carefully maintained in the aforementioned narrow range. Carbon dioxide is part of a prominent buffer system in the human body; it keeps the pH within the proper range. This buffer system involves carbonic acid (H2CO3) and bicarbonate (HCO3–) anion. If too much H+ enters the body, bicarbonate will combine with the H+ to create carbonic acid and limit the decrease in pH. Likewise, if too much OH– is introduced into the system, carbonic acid will rapidly dissociate into bicarbonate and H+ ions. The H+ ions can combine with the OH– ions, limiting the increase in pH. While carbonic acid is an important product in this reaction, its presence is fleeting because the carbonic acid is released from the body as carbon dioxide gas each time we breathe. Without this buffer system, the pH in our bodies would fluctuate too much and we would fail to survive.
Water has many properties that are critical to maintaining life. It is polar, allowing for the formation of hydrogen bonds, which allow ions and other polar molecules to dissolve in water. Therefore, water is an excellent solvent. The hydrogen bonds between water molecules give water the ability to hold heat better than many other substances. As the temperature rises, the hydrogen bonds between water continually break and reform, allowing for the overall temperature to remain stable, although increased energy is added to the system. Water’s cohesive forces allow for the property of surface tension. All of these unique properties of water are important in the chemistry of living organisms.
The pH of a solution is a measure of the concentration of hydrogen ions in the solution. A solution with a high number of hydrogen ions is acidic and has a low pH value. A solution with a high number of hydroxide ions is basic and has a high pH value. The pH scale ranges from 0 to 14, with a pH of 7 being neutral. Buffers are solutions that moderate pH changes when an acid or base is added to the buffer system. Buffers are important in biological systems because of their ability to maintain constant pH conditions.
Which of the following statements is not true?
D
Using a pH meter, you find the pH of an unknown solution to be 8.0. How would you describe this solution?
C
The pH of lemon juice is about 2.0, whereas tomato juice’s pH is about 4.0. Approximately how much of an increase in hydrogen ion concentration is there between tomato juice and lemon juice?
C
Why can some insects walk on water?
Some insects can walk on water, although they are heavier (denser) than water, because of the surface tension of water. Surface tension results from cohesion, or the attraction between water molecules at the surface of the body of water [the liquid-air (gas) interface].
Explain why water is an excellent solvent.
Water molecules are polar, meaning they have separated partial positive and negative charges. Because of these charges, water molecules are able to surround charged particles created when a substance dissociates. The surrounding layer of water molecules stabilizes the ion and keeps differently charged ions from reassociating, so the substance stays dissolved.

You can also download for free at http://cnx.org/contents/b3c1e1d2-839c-42b0-a314-e119a8aafbdd@12.1
Attribution: