By the end of this section, you will be able to:
In the early stages of embryonic development, the embryo’s skeleton consists of fibrous membranes and hyaline cartilage. By the sixth or seventh week of embryonic life, the actual process of bone development, ossification (osteogenesis), begins. There are two osteogenic pathways—intramembranous ossification and endochondral ossification—but bone is the same regardless of the pathway that produces it.
Bone is a replacement tissue; that is, it uses a model tissue on which to lay down its mineral matrix. For skeletal development, the most common template is cartilage. During fetal development, a framework is laid down that determines where bones will form. This framework is a flexible, semi-solid matrix produced by chondroblasts and consists of hyaluronic acid, chondroitin sulfate, collagen fibers, and water. As the matrix surrounds and isolates chondroblasts, they are called chondrocytes. Unlike most connective tissues, cartilage is avascular, meaning that it has no blood vessels supplying nutrients and removing metabolic wastes. All of these functions are carried on by diffusion through the matrix. This is why damaged cartilage does not repair itself as readily as most tissues do.
Throughout fetal development and into childhood growth and development, bone forms on the cartilaginous matrix. By the time a fetus is born, most of the cartilage has been replaced with bone. Some additional cartilage will be replaced throughout childhood, and some cartilage remains in the adult skeleton.
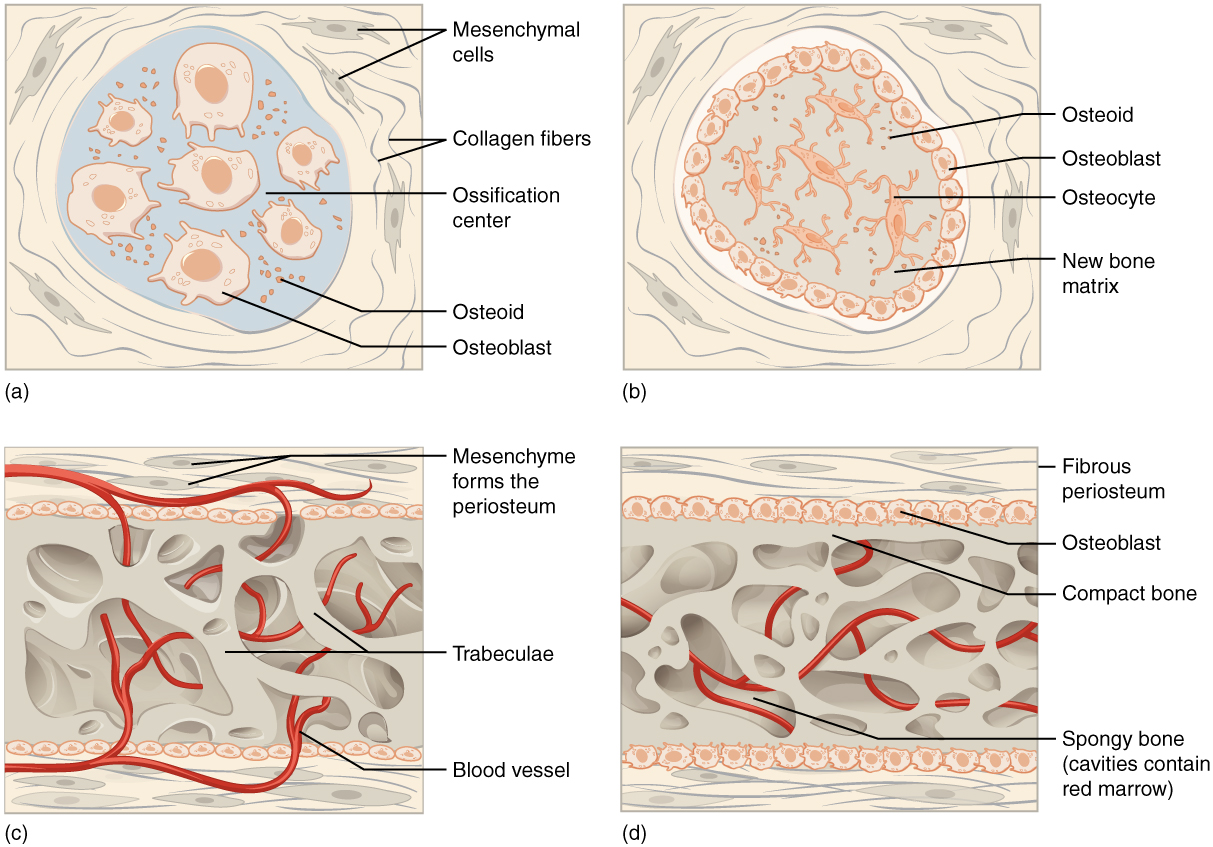
During intramembranous ossification, compact and spongy bone develops directly from sheets of mesenchymal (undifferentiated) connective tissue. The flat bones of the face, most of the cranial bones, and the clavicles (collarbones) are formed via intramembranous ossification.
The process begins when mesenchymal cells in the embryonic skeleton gather together and begin to differentiate into specialized cells ([link]a). Some of these cells will differentiate into capillaries, while others will become osteogenic cells and then osteoblasts. Although they will ultimately be spread out by the formation of bone tissue, early osteoblasts appear in a cluster called an ossification center.
The osteoblasts secrete osteoid, uncalcified matrix, which calcifies (hardens) within a few days as mineral salts are deposited on it, thereby entrapping the osteoblasts within. Once entrapped, the osteoblasts become osteocytes ([link]b). As osteoblasts transform into osteocytes, osteogenic cells in the surrounding connective tissue differentiate into new osteoblasts.
Osteoid (unmineralized bone matrix) secreted around the capillaries results in a trabecular matrix, while osteoblasts on the surface of the spongy bone become the periosteum ([link]c). The periosteum then creates a protective layer of compact bone superficial to the trabecular bone. The trabecular bone crowds nearby blood vessels, which eventually condense into red marrow ([link]d).

Intramembranous ossification begins in utero during fetal development and continues on into adolescence. At birth, the skull and clavicles are not fully ossified nor are the sutures of the skull closed. This allows the skull and shoulders to deform during passage through the birth canal. The last bones to ossify via intramembranous ossification are the flat bones of the face, which reach their adult size at the end of the adolescent growth spurt.
In endochondral ossification, bone develops by replacing hyaline cartilage. Cartilage does not become bone. Instead, cartilage serves as a template to be completely replaced by new bone. Endochondral ossification takes much longer than intramembranous ossification. Bones at the base of the skull and long bones form via endochondral ossification.
In a long bone, for example, at about 6 to 8 weeks after conception, some of the mesenchymal cells differentiate into chondrocytes (cartilage cells) that form the cartilaginous skeletal precursor of the bones ([link]a). Soon after, the perichondrium, a membrane that covers the cartilage, appears [link]b).
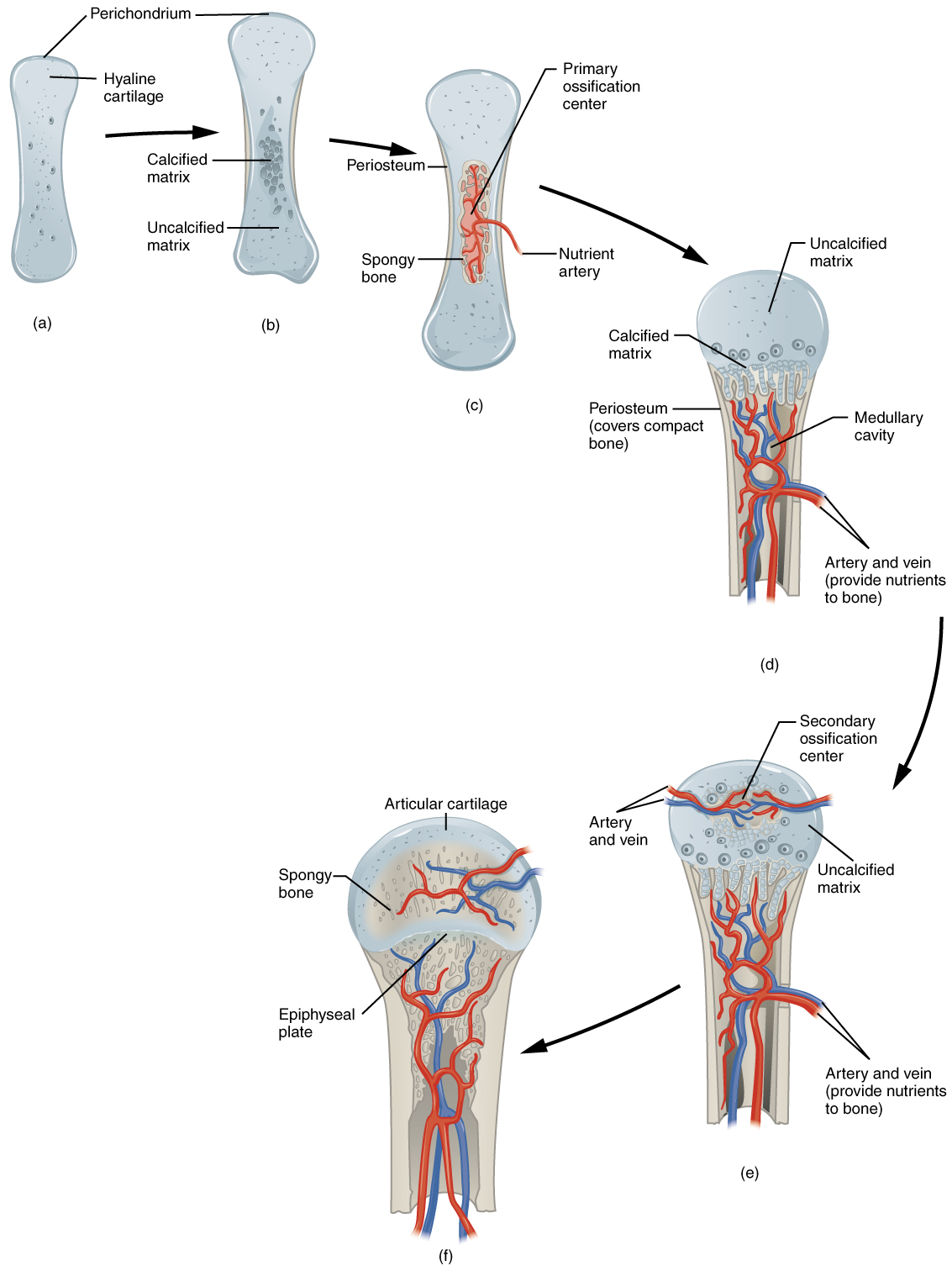
As more matrix is produced, the chondrocytes in the center of the cartilaginous model grow in size. As the matrix calcifies, nutrients can no longer reach the chondrocytes. This results in their death and the disintegration of the surrounding cartilage. Blood vessels invade the resulting spaces, not only enlarging the cavities but also carrying osteogenic cells with them, many of which will become osteoblasts. These enlarging spaces eventually combine to become the medullary cavity.
As the cartilage grows, capillaries penetrate it. This penetration initiates the transformation of the perichondrium into the bone-producing periosteum. Here, the osteoblasts form a periosteal collar of compact bone around the cartilage of the diaphysis. By the second or third month of fetal life, bone cell development and ossification ramps up and creates the primary ossification center, a region deep in the periosteal collar where ossification begins ([link]c).
While these deep changes are occurring, chondrocytes and cartilage continue to grow at the ends of the bone (the future epiphyses), which increases the bone’s length at the same time bone is replacing cartilage in the diaphyses. By the time the fetal skeleton is fully formed, cartilage only remains at the joint surface as articular cartilage and between the diaphysis and epiphysis as the epiphyseal plate, the latter of which is responsible for the longitudinal growth of bones. After birth, this same sequence of events (matrix mineralization, death of chondrocytes, invasion of blood vessels from the periosteum, and seeding with osteogenic cells that become osteoblasts) occurs in the epiphyseal regions, and each of these centers of activity is referred to as a secondary ossification center ([link]e).
The epiphyseal plate is the area of growth in a long bone. It is a layer of hyaline cartilage where ossification occurs in immature bones. On the epiphyseal side of the epiphyseal plate, cartilage is formed. On the diaphyseal side, cartilage is ossified, and the diaphysis grows in length. The epiphyseal plate is composed of four zones of cells and activity ([link]). The reserve zone is the region closest to the epiphyseal end of the plate and contains small chondrocytes within the matrix. These chondrocytes do not participate in bone growth but secure the epiphyseal plate to the osseous tissue of the epiphysis.
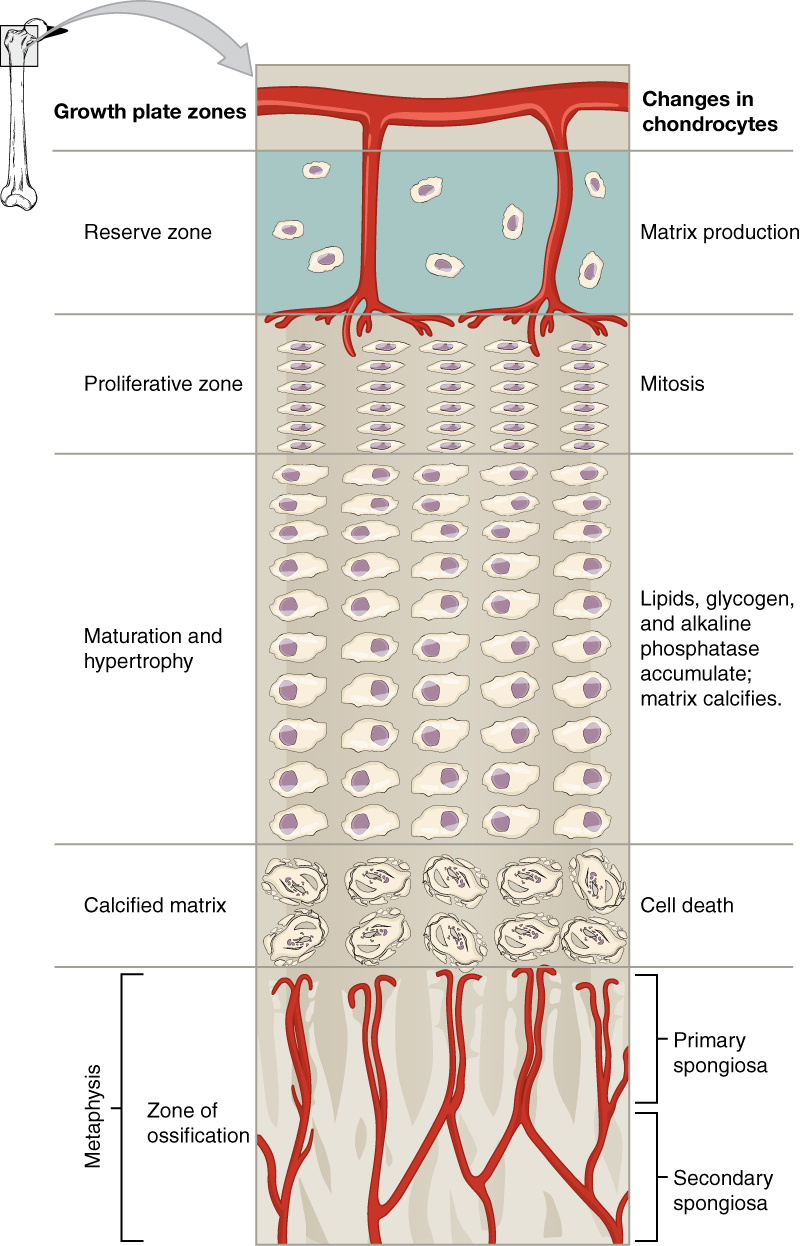
The proliferative zone is the next layer toward the diaphysis and contains stacks of slightly larger chondrocytes. It makes new chondrocytes (via mitosis) to replace those that die at the diaphyseal end of the plate. Chondrocytes in the next layer, the zone of maturation and hypertrophy, are older and larger than those in the proliferative zone. The more mature cells are situated closer to the diaphyseal end of the plate. The longitudinal growth of bone is a result of cellular division in the proliferative zone and the maturation of cells in the zone of maturation and hypertrophy.
Most of the chondrocytes in the zone of calcified matrix, the zone closest to the diaphysis, are dead because the matrix around them has calcified. Capillaries and osteoblasts from the diaphysis penetrate this zone, and the osteoblasts secrete bone tissue on the remaining calcified cartilage. Thus, the zone of calcified matrix connects the epiphyseal plate to the diaphysis. A bone grows in length when osseous tissue is added to the diaphysis.
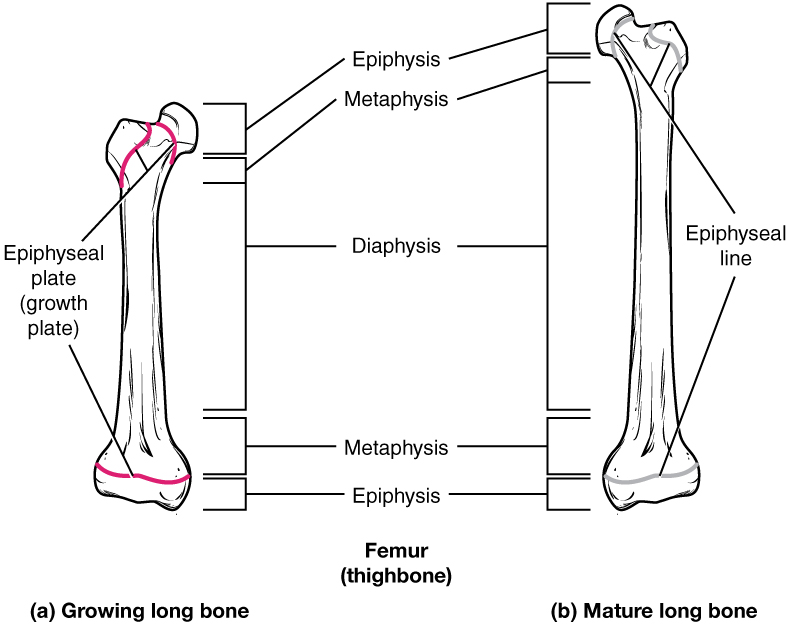
Bones continue to grow in length until early adulthood. The rate of growth is controlled by hormones, which will be discussed later. When the chondrocytes in the epiphyseal plate cease their proliferation and bone replaces the cartilage, longitudinal growth stops. All that remains of the epiphyseal plate is the epiphyseal line ([link]).
While bones are increasing in length, they are also increasing in diameter; growth in diameter can continue even after longitudinal growth ceases. This is called appositional growth. Osteoclasts resorb old bone that lines the medullary cavity, while osteoblasts, via intramembranous ossification, produce new bone tissue beneath the periosteum. The erosion of old bone along the medullary cavity and the deposition of new bone beneath the periosteum not only increase the diameter of the diaphysis but also increase the diameter of the medullary cavity. This process is called modeling.
The process in which matrix is resorbed on one surface of a bone and deposited on another is known as bone modeling. Modeling primarily takes place during a bone’s growth. However, in adult life, bone undergoes remodeling, in which resorption of old or damaged bone takes place on the same surface where osteoblasts lay new bone to replace that which is resorbed. Injury, exercise, and other activities lead to remodeling. Those influences are discussed later in the chapter, but even without injury or exercise, about 5 to 10 percent of the skeleton is remodeled annually just by destroying old bone and renewing it with fresh bone.
Skeletal System Osteogenesis imperfecta (OI) is a genetic disease in which bones do not form properly and therefore are fragile and break easily. It is also called brittle bone disease. The disease is present from birth and affects a person throughout life.
The genetic mutation that causes OI affects the body’s production of collagen, one of the critical components of bone matrix. The severity of the disease can range from mild to severe. Those with the most severe forms of the disease sustain many more fractures than those with a mild form. Frequent and multiple fractures typically lead to bone deformities and short stature. Bowing of the long bones and curvature of the spine are also common in people afflicted with OI. Curvature of the spine makes breathing difficult because the lungs are compressed.
Because collagen is such an important structural protein in many parts of the body, people with OI may also experience fragile skin, weak muscles, loose joints, easy bruising, frequent nosebleeds, brittle teeth, blue sclera, and hearing loss. There is no known cure for OI. Treatment focuses on helping the person retain as much independence as possible while minimizing fractures and maximizing mobility. Toward that end, safe exercises, like swimming, in which the body is less likely to experience collisions or compressive forces, are recommended. Braces to support legs, ankles, knees, and wrists are used as needed. Canes, walkers, or wheelchairs can also help compensate for weaknesses.
When bones do break, casts, splints, or wraps are used. In some cases, metal rods may be surgically implanted into the long bones of the arms and legs. Research is currently being conducted on using bisphosphonates to treat OI. Smoking and being overweight are especially risky in people with OI, since smoking is known to weaken bones, and extra body weight puts additional stress on the bones.
 Watch this video to see how a bone grows.
Watch this video to see how a bone grows.
All bone formation is a replacement process. Embryos develop a cartilaginous skeleton and various membranes. During development, these are replaced by bone during the ossification process. In intramembranous ossification, bone develops directly from sheets of mesenchymal connective tissue. In endochondral ossification, bone develops by replacing hyaline cartilage. Activity in the epiphyseal plate enables bones to grow in length. Modeling allows bones to grow in diameter. Remodeling occurs as bone is resorbed and replaced by new bone. Osteogenesis imperfecta is a genetic disease in which collagen production is altered, resulting in fragile, brittle bones.
Why is cartilage slow to heal?
C
Why are osteocytes spread out in bone tissue?
D
In endochondral ossification, what happens to the chondrocytes?
B
Which of the following bones is (are) formed by intramembranous ossification?
D
Bones grow in length due to activity in the ________.
A
Bones grow in diameter due to bone formation ________.
B
Which of the following represents the correct sequence of zones in the epiphyseal plate?
C
In what ways do intramembranous and endochondral ossification differ?
In intramembranous ossification, bone develops directly from sheets of mesenchymal connective tissue, but in endochondral ossification, bone develops by replacing hyaline cartilage. Intramembranous ossification is complete by the end of the adolescent growth spurt, while endochondral ossification lasts into young adulthood. The flat bones of the face, most of the cranial bones, and a good deal of the clavicles (collarbones) are formed via intramembranous ossification, while bones at the base of the skull and the long bones form via endochondral ossification.
Considering how a long bone develops, what are the similarities and differences between a primary and a secondary ossification center?
A single primary ossification center is present, during endochondral ossification, deep in the periosteal collar. Like the primary ossification center, secondary ossification centers are present during endochondral ossification, but they form later, and there are two of them, one in each epiphysis.

You can also download for free at http://cnx.org/contents/14fb4ad7-39a1-4eee-ab6e-3ef2482e3e22@11.1
Attribution: